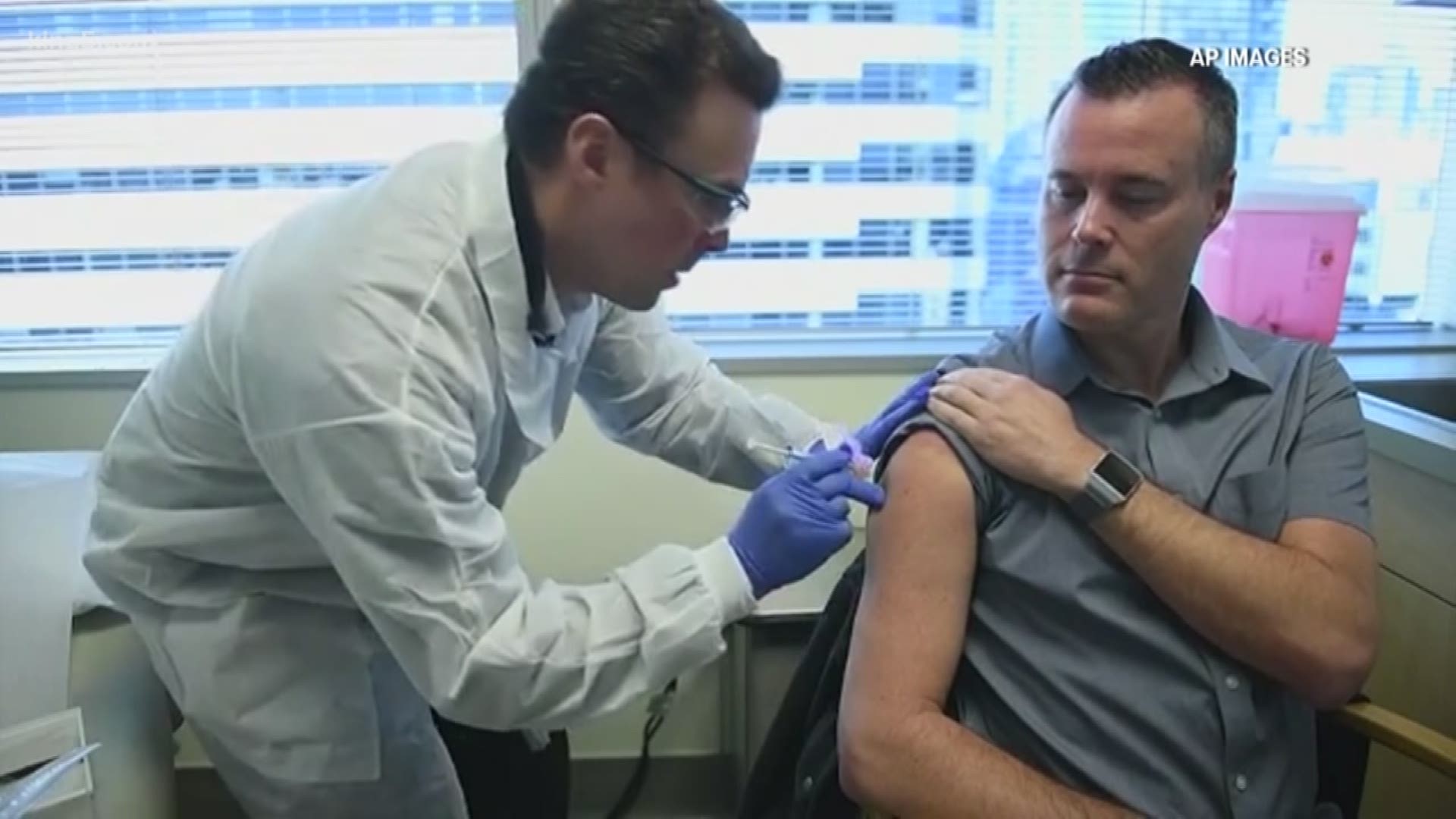
SEATTLE — U.S. researchers have given a healthy volunteer the first shot of an experimental coronavirus (COVID-19) vaccine as anxiously awaited testing opens.
Monday’s milestone is just the first step in a long process.
The National Institutes of Health (HIH) is funding the trial that is taking place at Kaiser Permanente Washington Health Research Institute in Seattle. Testing began with 45 young, healthy volunteers with different doses of shots co-developed by NIH and Moderna Inc.
Researchers started recruiting the 45 participants for the vaccine trial at the beginning of March. The goal of the first phase of the study is to learn about the vaccine’s safety and see how the immune system responds to it.
This vaccine, which is produced by ModernaTx, Inc., is not made from a weakened or killed version of coronavirus, and cannot cause infection, according to researchers. Instead, it’s made using a short segment of genetic code, which tells cells to make a protein found on the outside of the 2019 novel coronavirus. When cells create that protein, it triggers an immune response.
If someone is later infected with coronavirus, their immune system will theoretically kick in on overdrive, better helping fight the virus.
Public health officials said it will take a year to 18 months to fully validate any potential vaccine.
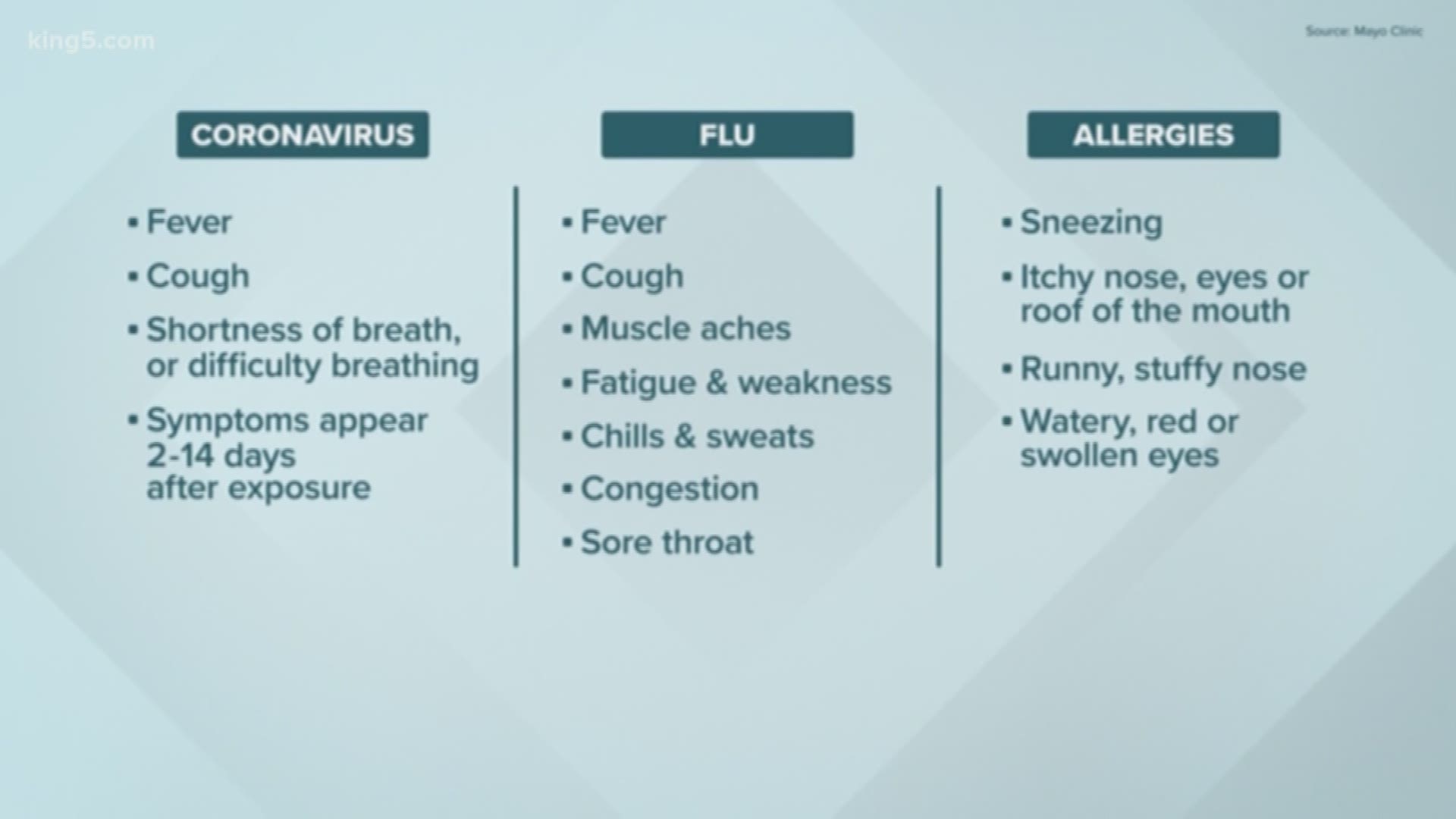
As of Monday morning, there were 769 confirmed cases of COVID-19 in Washington state, including 42 deaths, according to the Washington State Department of Health.