KNOXVILLE, Tenn. — When WBIR’s Robin Wilhoit decided to volunteer for an experimental COVID-19 vaccine trial, she wanted to document the process to provide a first-hand account.
She is participating in the trial run by pharmaceutical giant, Pfizer, through Volunteer Research Group at UT Medical Center.
"You know, I'm not nervous. I'm actually excited. I think we all feel helpless in this day and time, and the way I look at it is, this is just one little thing that I can do that will hopefully make a difference," Robin said before entering the medical center for her first visit.
The following is an account of what happens when you volunteer for a vaccine trial.
For more on Robin's vaccine trial experience: click here
Upon entering the medical center, you fill out a 25-page consent form, which explains the study, those who qualify and possible side effects, the most common being: soreness at the injection site and a fever that lasts for roughly 24 hours.
At this point, you move on to a temperature check, a health questionnaire, a vitals check to monitor blood pressure and heart rate and a blood test.
Then comes a nose swab coronavirus test. If the test comes back negative, you move on to a physical exam.
Once the doctors determine you have the green light to participate in the vaccine trial, it is a waiting game.
"It's a process. It takes a couple of hours," Robin said.
Pharmacists at the hospital then start preparing the vaccine and it becomes a waiting game. Each vaccine has five doses and is stored at -80 degrees. They must wait for the vaccine vial to thaw and for five participants to arrive so no dose is wasted.
"That does extend your stay, if you will, through this process. But the way I look at it is, it's worth every minute of it because in the end what we're doing is trying to find a vaccine that works," Robin said.
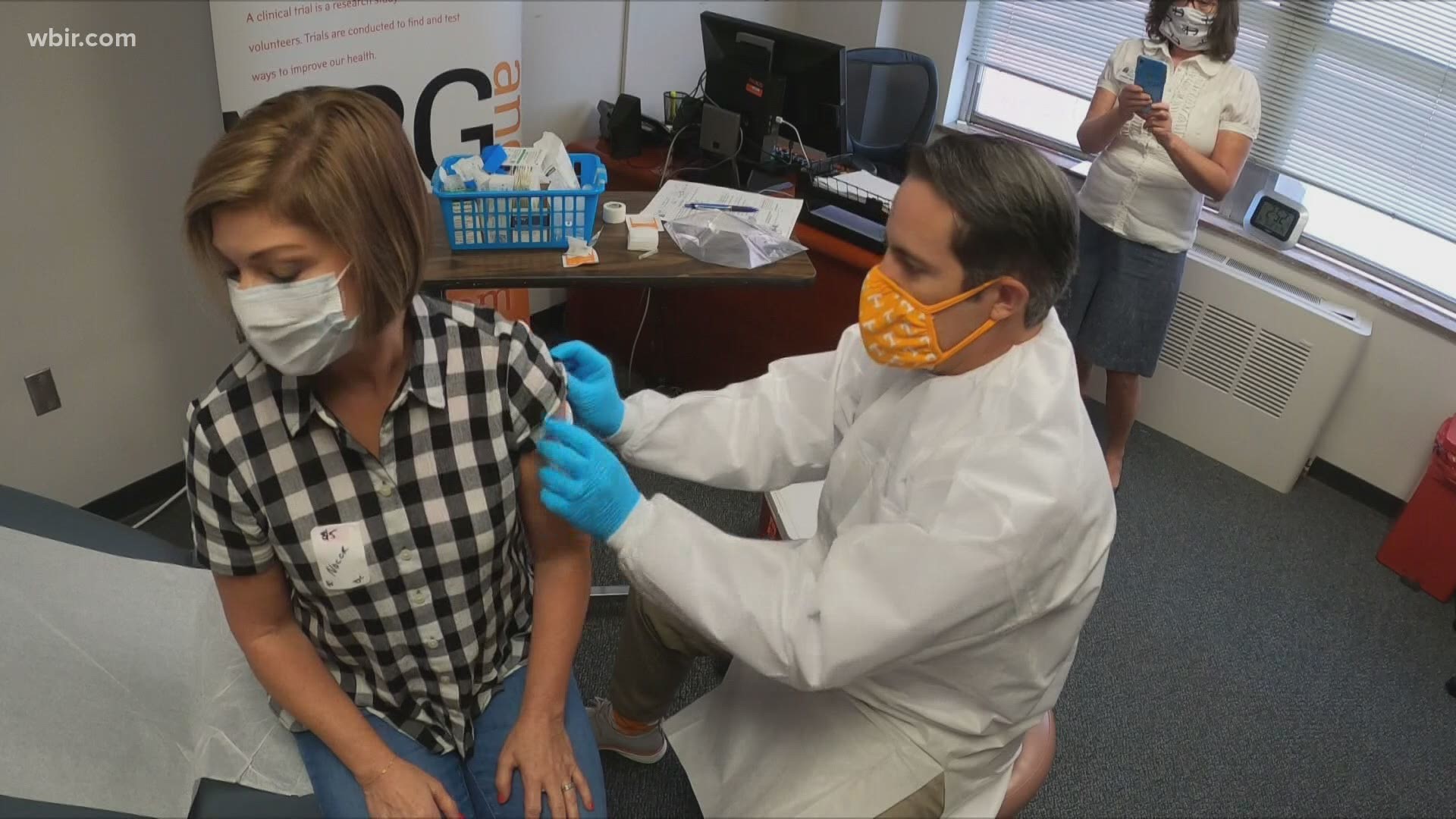
Each dose is randomized and each participant has a 50/50 chance of getting the experimental vaccine or a placebo injection. Since the trial is a double-blind study, only the pharmacist administering the vaccine knows whether the participant is receiving the real vaccine or the placebo.
Once the injection is ready, a pharmacist administers the vaccine to the participant.
It is important to note that the vaccine does not contain the coronavirus so you will not be injected with the virus.
"There was a little pinch, if you will, and a little tingly I felt as it was going forth," Robin said shortly after getting the vaccine.
More information on this particular type of vaccine can be found here:
You then wait for 30 minutes under observation to make sure you do not have an adverse reaction to the injection.
During the wait, you are given instructions for the next part of the study. Every day for the next week at 8 p.m. you fill out a diary through an app on your phone.
In this diary, you record your temperature and any potential side effects using a kit provided by the hospital containing a thermometer and paper wheel that can be used to measure any swelling at the injection site.
You also have a contact number for nurses at the medical center who can be reached 24/7 if you start showing any symptoms of COVID-19.
For the next week, you monitor your body’s response to the injection and record it on the app.
Robin said her only side effect after her first dose was a little tenderness at the injection site.
"It's not so sore that it's going to keep me from doing my daily activities by any means, but it's not a situation where I'm gonna want to go out and lift weights or anything like that," Robin said in a video diary the morning after her first shot.
She said the soreness was gone within a day.
After roughly a month, you will be scheduled for the second and final dose of the trial.