KNOXVILLE, Tenn. — As they report nearly full intensive care units, Knox County hospitals confirmed Wednesday they have begun distributing an experimental COVID-19 treatment for people who are at high risk of needing hospitalization from the virus.
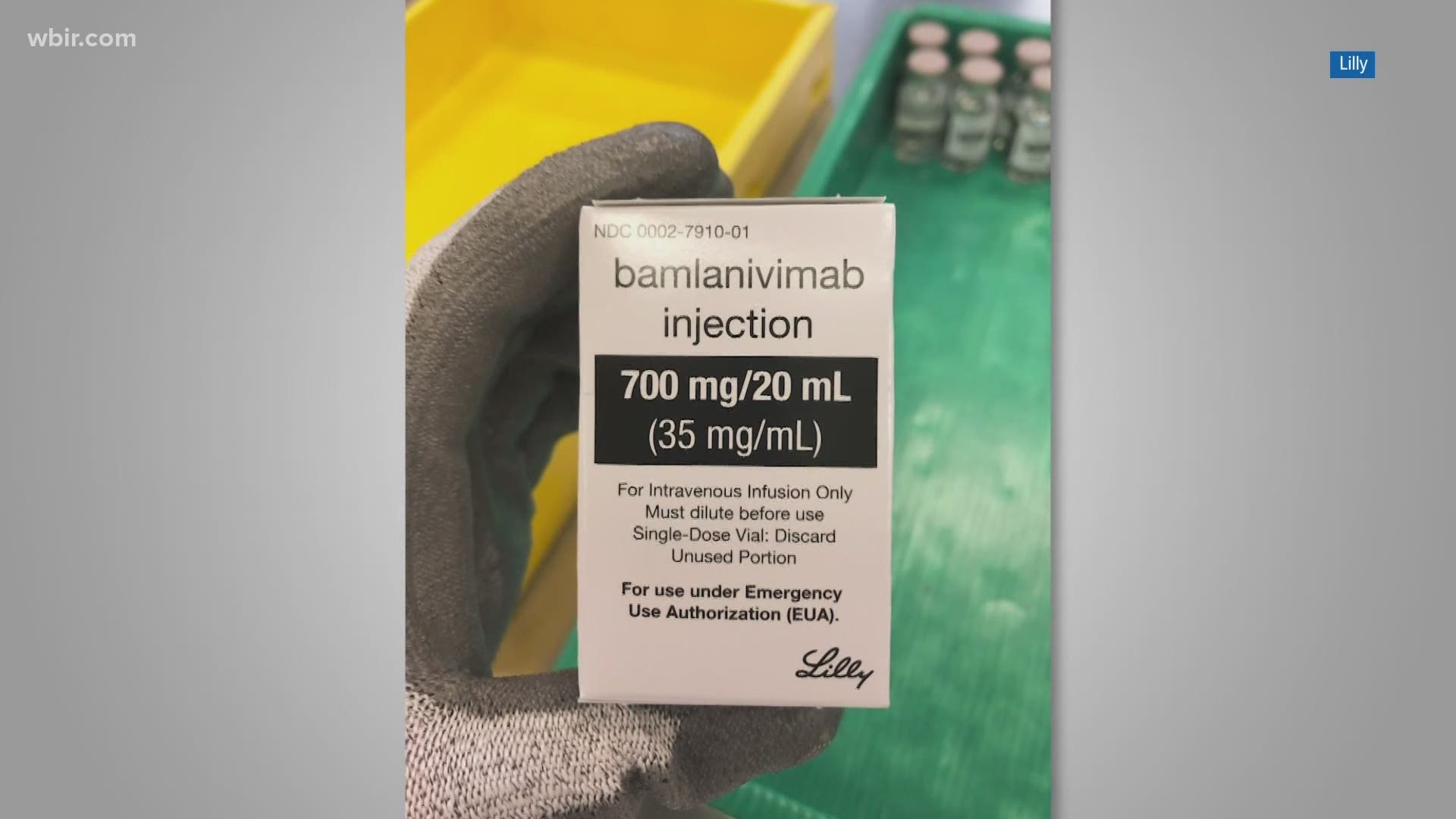
In a joint statement to 10News, the University of Tennessee Medical Center, Tennova Healthcare and Covenant Health announced each hospital began on Tuesday to administer bamlanivimab, which received emergency authorization from the FDA on November 9.
The drug, which is administered through an IV, will be given to some people who have tested positive for the coronavirus and are either older than 65 or obese, as determined by body mass index, the release said.
The drug is not authorized to treat people who are already in the hospital because of COVID-19. The hospitals said they would not use it for people who require supplemental oxygen.
The hospitals said eligible patients must have tested positive for COVID-19, be within seven days of symptom onset, and receive a doctor's referral for the outpatient treatment.
"Bamlanivimab works to prevent COVID‐19 from spreading further in a patient’s body," the joint statement said.
The FDA authorized the drug for people with chronic kidney disease and diabetes, as well as some patients with high blood pressure and chronic respiratory problems.
However, the Knox County hospitals have implemented more narrow eligibility guidelines that cut out some patients the FDA said could qualify.
"I don’t understand why everyone can’t get it," Marcus McNay, 53 said. "That’s my biggest concern."
McNay, a salesman from LaFollette who has diabetes and a history of pneumonia, said his doctor told him he would qualify for a monoclonal antibody treatment similar to bamlanivimab after he tested positive for COVID-19, but that he didn't meet Knox County's more restrictive criteria.
"It was like a gut punch actually," McNay said about finding out he couldn't get the drug. "It was like somebody knocked me in the gut and took the breath out of me. Somehow in East Tennessee the 'powers that be' said only morbidly obese people and people over 65 with underlying health conditions can get this treatment."
According to a rough estimate from the CDC's BMI calculator, a 5'8 adult who weighs more than 230lbs. has a BMI of at least 35. This calculation may vary between individuals.
Bamlanivimab is currently in phase two clinical trials and has not yet been approved by the FDA. It has been authorized for emergency use in an attempt to stop patients from getting so sick they need hospitalization in high numbers, potentially overwhelming the medical system.
"Hospitalizations and emergency room visits occurred in 3% of bamlanivimab-treated patients on average compared to 10% in placebo-treated patients," the FDA said in its emergency authorization release.
The emergency authorization was based on an interim analysis of 465 people.
The FDA said the "known and potential benefits outweigh the known and potential risks for the drug.”